May 17, 2012 | Health Matters
FDA panel backs home tests for HIV
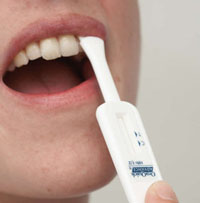 US citizens are one step closer to being able to test themselves for HIV in the privacy of their own homes. This week, the Food and Drug Administration advisory panel recommended approval of the first rapid, over-the-counter HIV test.
US citizens are one step closer to being able to test themselves for HIV in the privacy of their own homes. This week, the Food and Drug Administration advisory panel recommended approval of the first rapid, over-the-counter HIV test.
All 17 members of the panel agreed there were more benefits than risks with the OraQuick HIV test. The mouth swab test returns a result within 20 minutes; however, it is not 100% accurate. It was found to accurately indicate the virus only 93% of the time when administered at home (versus 99% when the same test is used by professionals).
Government estimates state that about 1.2 million HIV carriers in the U.S. are not even aware they are infected. The panel felt that the test could help prevent 4,000 new transmissions of the virus each year.
"Over-the-counter testing has the potential to reach a far greater number of people who want to know their HIV status on their own terms," said Tom Donohue, founder of HIV awareness group Who's Positive.
Currently the test is being sold to health care professionals for $17.50. The company would not disclose the cost of the proposed consumer version.
The FDA will now consider the panel's finding and make a final decision on the test later this year.
Do-It-Yourself HIV Test? FDA Considers Approval [TIME]
OraQuick, Take-Home HIV Test, Backed By FDA Panel [Huffington Post]
Gay Porn Reviews
Collegiate
86 Broke Str8 Boys [visit]91 Corbin Fisher [visit]
93 Bel Ami Online [visit]
88 Blake Mason [visit]
88 Cocky Boys [visit]
▻ All Collegiate Reviews
Hunks
84 The Bro Network [visit]79 Men.com [visit]
81 Active Duty [visit]
87 Chaos Men [visit]
85 Next Door Studios [visit]
85 Falcon / HH [visit]
82 Pride Studios [visit]
88 Lucas Ent. [visit]
80 Fucker Mate [visit]
▻ All Hunks Reviews
Masculine
84 Raging Stallion [visit]87 Tim Tales [visit]
82 Pride Studios [visit]
79 Butch Dixon [visit]
78 Titan Men [visit]
88 Lucas Ent. [visit]
▻ All Masculine Reviews
Twinks
88 Freshmen [visit]83 Boyfun [visit]
82 Carnal Plus [visit]
79 Fun Size Boys [visit]
▻ All Twinks Reviews
Euro
88 Freshmen [visit]93 Bel Ami Online [visit]
83 Kristen Bjorn [visit]
▻ All Euro Reviews
Fetish
86 Fisting Inferno [visit]83 Serious Bondage [visit]
82 Kink Men [visit]
88 Lucas Ent. [visit]
▻ All Fetish Reviews
Networks
79 Men.com [visit]85 Next Door Studios [visit]
82 Pride Studios [visit]
▻ All Networks Reviews
Top | Home | About Us | Contact Us | Reviews | Galleries | News | What's Up?
BananaGuide: the gay man's guide to porn
© 2000, 2024 Untangled Web Inc.
